what does lac on oxogen do to the brain
Increased blood lactate levels: a marker of...?
1. Lactate metabolism
Lactate is a normal stop production of glycolysis (Fig. 1). It tin can only be formed from pyruvate mediated by lactate dehydrogenase [i].
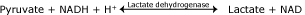
Equation 1
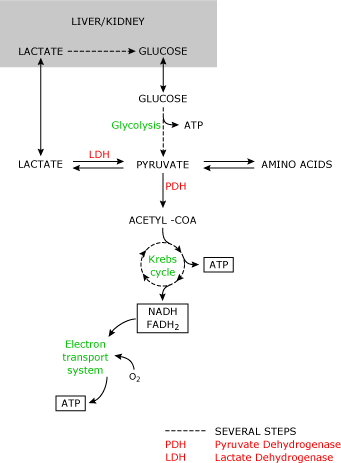
FIG. 1
Under normal conditions this reaction results in a lactate-to-pyruvate ratio of 10:1. All cells are capable of producing lactate. Tissues with a loftier metabolic rate (gut, encephalon, skeletal muscle) contribute largely to the daily lactate product.
Normal claret lactate levels are 1.3 mmol/L [2]. Lactate metabolism mainly occurs in the liver and kidney. Lactate tin can only be metabolized past the conversion to pyruvate. Therefore, blood lactate levels depend on pyruvate metabolism.
The irreversible conversion of pyruvate to Acetyl-CoA (mediated by pyruvate dehydrogenase) that is subsequently metabolized in the Krebs cycle results in the production of adenosine triphosphate (ATP), carbon dioxide and water.
ATP can be seen as a universal free energy source that is required in many vital cellular functions. Pyruvate can also exist used to regenerate glucose by the conversion to oxaloacetate.
In this way lactate can be converted back to glucose that tin can subsequently be converted to lactate (Cori cycle). Regeneration of glucose from lactate is an of import mechanism in restoring glucose levels and removal of lactate from the systemic apportionment post-obit prolonged tissue hypoxia (due east.g. diving mammals, cardiac abort) [3]. Last, pyruvate can exist converted to alanine and α-ketoglutarate.
The reverse of this reaction regenerates pyruvate that can thus exist used for oxidation or gluconeogenesis. From equation 1 we tin can conclude that even in the presence of a stable redox land (NAD-to-NADH ratio) and cellular pH, lactate levels will rise whenever the formation of pyruvate exceeds its utilization or the conversion of pyruvate to Acetyl-CoA is limited.
Utilization of pyruvate may decrease when pyruvate dehydrogenase is deficient (inborn error of metabolism).
Dysfunction of the pyruvate dehydrogenase complex may occur during sepsis, resulting in increased pyruvate levels and hence claret lactate levels [4]. The clinically most relevant crusade of limited pyruvate utilization is a cellular lack of oxygen.
Both oxidation of pyruvate and gluconeogenesis require the presence of oxygen. Consequently when oxygen levels autumn, glucose is mainly converted to lactate.
Although this reaction also produces ATP, it is less effective (2 molecules of ATP versus 34 molecules of ATP when metabolized in the Krebs bicycle).
Three pathways are involved in the send of lactate across the cell membrane [v]. First, free improvidence of lactic acid plays a role in lactate efflux and uptake by the cells, specially at college concentrations.
Second, exchange for another anion such as Cl- or HCOthree - facilitates lactate ship across the cell membrane.
The 3rd and most important pathway involves an H+-linked carrier mechanism (monocarboxylate transporter). This H+-linked transporter increases lactate flux in the presence of a pH gradient beyond the membrane.
By this mechanism lactate uptake by the cell (east.g. skeletal muscle and cardiac myocytes) is increased during acidosis. In contrast, lactate efflux will increase during alkalemia, resulting in an increment in lactate levels during systemic alkalemia [half dozen].
The increment in lactate levels during alkalemia could besides be related to the stimulation of phosphofructokinase in these conditions, resulting in increased glycolysis and thus increased lactate production.
Although increased blood lactate levels are frequently associated with the presence of a metabolic acidosis (lactic acidosis) the production of lactate does non result in net hydrogen ion (H+) product as the H+ ions are reutilized in the product of ATP from adenosine diphosphate (ADP) and adenosine monophosphate (AMP).
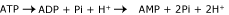
Equation 2
The inability of the cells to reutilize H+-ions generated by the hydrolysis of ATP during hypoxia is mainly responsible for the metabolic acidosis in these conditions [7].
2. Relationship betwixt lactate and tissue hypoxia
Clinically, blood lactate levels are frequently used to monitor tissue hypoxia. The rationale for this, as outlined higher up, is clear: The utilization of pyruvate depends on the presence of oxygen so that decreases in cellular oxygen delivery should result in increased lactate production and thus blood lactate levels (Fig. 2A).
Tissue hypoxia is best defined every bit the presence of an imbalance between the demand for oxygen and the actual delivery of oxygen (Do2). Equally Practisetwo decreases, tissues maintain their oxygen utilization to meet their oxygen demand by extracting more than oxygen.
An increase in the calculated oxygen extraction ratio (O2ER) and a decrease in the mixed venous oxygen saturation usually reflect this. Global DOii is a role of the arterial oxygen content (ctOtwo) and the cardiac output (Q̇t) (equation 3).
![]()
ctHb = hemoglobin level
sOii(a) = arterial oxygen saturation
Equation three
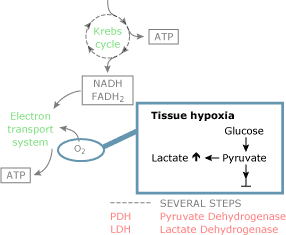
FIG. 2A
Although a decrease in each of the components can crusade a subtract in Practice2, decreases in hemoglobin levels and arterial oxygen saturation are commonly accompanied by compensatory increases in cardiac output so that DO2 tin be maintained to see oxygen demand [eight] and thus generally do not result in tissue hypoxia [nine].
When this compensatory mechanism fails, Exercise2 volition subtract more speedily in these conditions [10].
When DO2 falls below a critical level, oxygen need can no longer be met and oxygen consumption starts to fall coincided with an increment in blood lactate levels (Fig. 1).
This phenomenon (supply dependency) has been demonstrated during experimental decreases in hemoglobin levels, arterial oxygen saturation and cardiac output [11-thirteen]. For obvious reasons, effects of lowering Practiceii to critical levels in humans are not well studied.
In patients and good for you volunteers, acute decreases in hemoglobin levels are met by compensatory increases in cardiac output to maintain tissue oxygen delivery [8]. In 2 studies, Shibutani, Komatsu and co-workers showed that in cardiac surgery patients supply dependency occurred when Doii was below a critical level of 300 mL/min [fourteen,15].
In patients with increased claret lactate levels, oxygen consumption fell immediately when DOtwo was lowered [14].
Ronco et al [16] also showed that lowering DO2 below a critical level resulted in a decrease in oxygen consumption and a rise in claret lactate levels.
In critically sick patients, Vincent et al [17] showed that oxygen consumption increased only in patients with increased blood lactate levels when Dotwo was increased past the infusion of dobutamine. Interpretation of increased blood lactate levels in patients with sepsis tin exist hard [seven].
However, in the early phase of septic daze, increased blood lactate levels take as well been associated with the presence of supply dependency and thus tissue hypoxia [18]. Should we therefore conclude that in clinical exercise, increased blood lactate levels should be regarded every bit an indicator of the presence of tissue hypoxia [19]?
3. Other causes of increased lactate levels
In the absence of cellular hypoxia, dysfunction of the PDH enzyme complex also results in an increase in pyruvate levels (Fig. 2B). Increased aerobic glycolysis increases intracellular pyruvate levels when there is no need for increased ATP production (i.e. when oxygen need is not increased).
Increased activity of the Na+-K+-ATPase in the presence of cellular normoxia has been related to this pathway of aerobic lactate product [twenty]. Protein breakdown results in an increased amino acid disposal that may increase pyruvate levels in the process of gluconeogenesis (Fig. 2C).
Increased lactate production in the absenteeism of cellular hypoxia has been documented in clinically relevant experimental settings (sepsis, chatecholamine treatment) and in patients [21,22]. Dysfunction of the PDH enzyme has been documented in experimental and clinical sepsis and could besides be related to the decreased lactate clearance in septic patients.
In addition, decreased blood flow (decreased commitment of lactate) to liver and kidney could influence lactate clearance. Finally, persistent cellular hypoxia in the presence of a hemodynamically stable condition could exist related to decreased clearance (Fig. 2d).
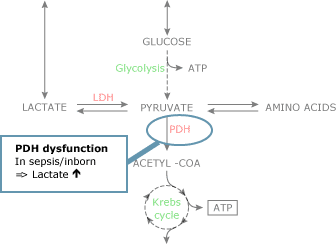
FIG. 2B.
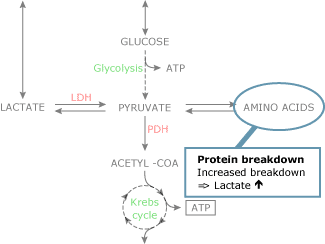
FIG. 2C.
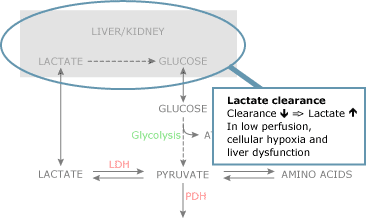
FIG. 2D.
Plasma levels of lactate non only upshot from the production of the molecule merely also from its clearance. Lactate clearance occurs predominantly in the liver and kidney, whereas during hyperlactatemia muscles also metabolize lactate.
Decreased lactate clearance rather than increased production could be an of import cause of increased blood lactate levels in septic patients following hemodynamic stabilization [23]. Notwithstanding, also in patients following cardiac surgery and in patients with liver dysfunction, clearance of blood lactate is deranged [24,25].
iv. Lactate and acidosis
The relationship betwixt lactate and hydrogen ion concentration is far from straightforward. Contempo reviews have stressed that, especially in sepsis, the product of lactate and H+-ions tin can be unrelated [vii]. In addition, concrete chemistry has stressed the importance of the stiff-ion difference as an important crusade of changes in H+-ion concentration and gratis water as the major source of these H+-ions [26].
Although the relationship between lactate levels and indices of metabolic acidosis are reasonable in low-flow states, the product of unmeasured ions, the presence of renal dysfunction and the arterial pCO2 (simply influenced past manipulating the mechanical ventilator) are probably related to the weak-to-absent correlation between arterial pH, base of operations excess or base deficit and lactate levels in the full general intensive intendance population and patients with sepsis in particular [27,28].
Groeneveld et al [29] already showed that despite similar lactate levels, patients with septic stupor had a lower arterial pH than patients with non-septic shock. In this study the severity of sepsis, equally related to ultimate survival, also influenced the relationship between lactate level and arterial pH.
v. Lactate levels in clinical exercise
From the to a higher place nosotros can conclude that increased blood lactate levels with or without concomitant acidosis reflect a complex metabolic disturbance in which increased aerobic and anaerobic production and decreased clearance are important elements.
Furthermore, the importance of these elements differs in different disease states. It is therefore no surprise that the reflection of such a complex metabolic disturbance is not met past clinical signs of disquisitional illness, including the classical signs of shock [30].
Likewise, other laboratory abnormalities, usually coinciding disquisitional illness, practise not reverberate blood lactate levels or changes in blood lactate levels [28,31]. In addition, a given level of oxygen commitment or oxygen consumption besides cannot predict the presence of oxygen supply dependency or increased blood lactate levels [32]. This underlines the importance of measuring lactate levels rather than estimating them from other (biochemical) variables.
v.1 Measurement techniques and sampling site
When first described (Gaglio 1886), the measurement of lactate levels required the collection of 100-200 mL blood and took several days to complete. In 1964, Broder and Weil [33] were the first to use a photospectrometric method to measure lactate levels in whole blood, decreasing turnaround time substantially.
With this they set up a trend in the monitoring of blood lactate levels in critically ill patients. The labor-intensive attribute of the early on measurement technique limited the widespread use, equally results were unremarkably bachelor long (hours) afterwards therapeutic decisions had to be fabricated.
The availability of a substrate-specific electrode now enables the clinician to measure lactate concentrations rapidly (within two minutes) in a minimal corporeality (130 µL) of plasma or whole claret, using a blood gas analyzer. Using a device similar this can simultaneously provide information on hemoglobin levels, oxygen saturation and blood lactate levels.
Recently a handheld device using a cogitating photometry method has been introduced and validated in emergency department patients [34] and ICU patients [35].
With this handheld device blood lactate measurements tin be fabricated using one driblet of whole blood, and results are available within 60 seconds. This relatively cheap and like shooting fish in a barrel-to-perform method can exist used for rapid lactate measurements in emergency situations [36].
In the intensive care unit of measurement, claret drawn from an arterial line is normally used to measure claret lactate levels. All the same, (mixed) venous blood tin also be used to measure claret lactate levels in critically ill patients [37,38].
When capillary or peripheral venous blood is used, damming of blood and muscle activity should be avoided [39].
Nerveless blood samples should be stored on ice and measurements of blood lactate levels should be performed quickly when metabolism of red and white blood cells is not stopped by the add-on of east.g. fluoride to the sample [40].
5.2 Increased lactate in relation to clinically relevant endpoints
Lactate monitoring needs to exist incorporated into an interventional and therapeutic programme in order for the patient to benefit from these measurements. Could such an easily obtainable parameter of circuitous abnormalities and so be used to assign patients to an interventional plan?
More specifically, could lactate levels be used to identify patients that would benefit from intensive care admission?
For this, lactate levels should exist related to risk of morbidity and mortality from a disease land that is best managed in an intensive care unit.
For more than 25 years, blood lactate levels have discriminated patients with less morbidity from patients with more morbidity and survivors from non-survivors in many forms of surgical interventions, trauma and disquisitional affliction [33,41-45]. Too, most patients with increased lactate levels have a loftier take chances of compromised vital organ functions [2].
In improver, express clearance of lactate in patients without circulatory failure is still related to increased bloodshed in patients with sepsis [46]. Although the relationship betwixt lactate and acidosis is circuitous, the combination of increased lactate levels and acidosis bears a high mortality [44].
Some studies showed that in specific patient groups, lactate levels were better predictors of survival and development of organ failure than circuitous scoring systems similar APACHE II [47].
In a contempo study, Smith et al [48] showed that blood lactate levels could discriminate patients with loftier risk of morbidity and mortality from patients with relative low adventure.
The portability of the lactate measuring devices, the ease and speed of the measurements could thus be important factors in the clinical utility of lactate levels as indicators for intensive care admission.
5.3. Treatment of increased lactate levels
Correction of hyperlactatemia by increasing the metabolism of pyruvate and hence lactate has no significant effect on mortality in critically sick patients. Dichloroacetate enhances the activity of the pyruvate dehydrogenase circuitous, thus decreasing claret lactate levels.
Both experimental and clinical studies have shown that administration of dichloroacetate decreases blood lactate levels during sepsis and septic shock [49,50,fifty].
However, in a recent controlled clinical trial (252 patients), assistants of dichloroacetate in critically ill patients with hyperlactatemia and metabolic acidosis had no significant effect on hemodynamics and survival [51].
As well correction of the metabolic acidosis accompanying hyperlactatemia has not been shown to amend hemodynamics, tissue hypoxia and survival in critically ill patients [52,53].
The mainstay of therapy in patients with increased claret lactate levels is improvement of tissue oxygen delivery. This is commonly accomplished past increases in DO2. Fluid resuscitation, hemoglobin substitution and maintenance of arterial oxygen saturation are frequently used to amend tissue oxygen delivery.
Even so, fluid resuscitation and inotropes to increase cardiac output have consistently been plant to ameliorate tissue oxygen delivery in patients with tissue hypoxia and thus remain the mainstay of therapy in these circumstances [54-58].
Few studies take prospectively studied the effect of using lactate levels to guide therapy. Blow et al [59] vigorously resuscitated trauma patients with persistent hyperlactatemia to normalize blood lactate levels.
Patients who failed to normalize lactate levels within 24 hours had a poor upshot whereas with patients in whom blood lactate levels returned to normal within 24 hours issue was improved when compared with a historic control group.
Similar findings have been reported by others. In a prospective randomized study of goal-oriented hemodynamic therapy in cardiac surgery patients aimed at maintaining normal claret lactate levels, Pölönen et al [60] showed that patients in the protocol grouping had lower infirmary stay and mortality than patients in the control grouping.
5.4. Clinical application of lactate measurements
A measurement of claret lactate should be part of the evaluation of every critically sick patient unless the diagnosis is obvious and immediate intervention (surgery) is necessary (like in ruptured aneurysm). Especially in the early stages of critical affliction, increased blood lactate levels indicate tissue hypoxia and insufficient compensatory mechanisms.
A deplorable but illustrative case will clarify this. A young trauma victim was admitted to our emergency department with fractures of his upper and lower legs. He was conscious, acceptable and oriented in time, place and persons.
He was hemodynamically stable with a blood pressure of 130/eighty and a eye rate of 115 b/min. Blood was drawn for routine evaluation including a blood lactate level. An echo of the abdomen was immediately performed and showed no abnormalities.
Within 15 minutes of inflow the patient developed a circulatory collapse and subsequent arrest.
CPR was ineffective and the patient died in the ER. Minutes after he died the laboratory results showed a claret lactate level on inflow of 15 mmol/50. Despite his adequate conscious and stable hemodynamics this patient was running out of compensatory mechanism and from this lactate level information technology was clear that something dramatic was about to happen.
In improver to the initial measurement of blood lactate levels, subsequent measurements inform the treating md on the adequacy of the resuscitation [61]. Persisting increased claret lactate levels or a failure to decrease lactate levels should exist followed by increasing oxygen delivery.
This could be achieved past increasing cardiac output [62,63] or microcirculatory claret flow with vasodilating agents like prostacyclin [64] or nitroglycerin [65].
In postoperative intendance we measure out lactate levels routinely and increase oxygen delivery whenever lactate levels rise above two.0 mmol/50, or when levels are increased upon admission to the intensive care [lx].
6. Decision
Increased blood lactate levels serve well as a marker of a complex metabolic derangement related to increased production, decreased clearance or a combination of both. Clinicians should understand these circuitous processes, capeesh the usefulness and the limitations of monitoring claret lactate levels.
Measurements of blood lactate levels should never replace a complete clinical evaluation. Rather, adding lactate measurements to clinical judgment and easily obtainable clinical variables enhances its predictive power [66,67].
The presence of increased blood lactate levels, especially in combination with acidosis, should urge the clinician to restore a probable imbalance betwixt oxygen demand and oxygen commitment to the tissues, as this is the most frequent cause of increased blood lactate levels.
Increased lactate levels take been consistently associated with morbidity and mortality in a wide range of disease states for many years.
Therefore the presence of increased blood lactate levels should prompt the clinician to initiate both diagnostic and immediate therapeutic deportment and intensive intendance admission should be considered.
Disclaimer
May comprise information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
Source: https://acutecaretesting.org/en/articles/increased-blood-lactate-levels-a-marker-of
0 Response to "what does lac on oxogen do to the brain"
Postar um comentário